By Johanna Schultz
When faced with a big problem, it’s often the small ideas that are able to create big results. Allowing those ideas to grow is equally important, according to Dr. Jacqueline Quinn, who helped create an innovative groundwater treatment technology that uses nanosize and microscale bits of iron (ZVI particles). “Let the science expand beyond your original plans,” says Dr. Quinn, “and it will take you places.” The result, Emulsified Zero-Valent Iron (EZVI), worked so well that it is currently in use to treat not only groundwater pollution but above-ground PCB pollutants as well.
Dr. Quinn, an environmental engineer in the Applied Sciences Division of the Kennedy Applied Technology Directorate, joined Kathleen Brooks, an analytical chemist in the center’s Materials Science Laboratory of the Center Operations Directorate, along with Drs. Christian Clausen, Cherie Geiger, and Debra Reinhart at the University of Central Florida’s Departments of Chemistry and Civil Environmental Engineering to come up with a solution that would provide a safe, effective, and economical way to clean dense nonaqueous phase liquids (DNAPLs) from the environment. DNAPLs are a common cause of environmental contamination at thousands of Department of Energy, Department of Defense, NASA, and private industry facilities around the country. Kennedy Space Center’s Launch Complex 34 was polluted with chlorinated solvents that were used to clean Apollo rocket parts during the space program’s early years. When left untreated, DNAPLs contaminate fresh water sources and are costly and difficult to remove.
The team’s original treatment concept relied on using iron, which has been used to eliminate chlorinated contaminants in groundwater for about a decade. Iron is cheap and is found in most groundwater environments from natural sources such as iron oxides, hydroxides, and sulfates or sulfides. It is also essentially nontoxic. When iron metal is exposed to chlorinated solvents (particularly dissolved solvents) in groundwater, it creates a reaction that replaces chlorine in the molecules with hydrogen, leaving a harmless end product of ethene or ethane.
“Once we came up with this idea, we saw that it could be extrapolated to treat a different compound in a different environment,” says Dr. Quinn. The expanded idea included treating polychlorinated biphenyls (PCBs), a compound commonly used in the 1970s for hundreds of applications from carbon paper to paints, adhesives, and dialectic transformers, which has proven exceptionally difficult to safely remove from the environment. “We have altered the metal we use for the degradation and developed different liquid membranes for the emulsion droplet to extend EZVI’s capabilities and treat PCBs in addition to DNAPLs,” explains Dr. Clausen. According to Dr. Quinn, “We’ve been able to take our original technology into a completely different application use.”
Leadership and vision were essential elements to the team’s success with EZVI. Brooks, who initially joined the team as a graduate student in environmental chemistry, found particular inspiration from the guidance and examples of the team’s senior researchers. Quinn also attributes EZVI’s success to the team’s collaborative and flexible approach to research. The research team had worked together on projects in the past and was “a very comfortable working group,” explains Dr. Quinn. “We know each other’s triggers and how to work with those sensitivities.” This familiarity promoted rapport, trust, and experimental freedom that were essential to thinking beyond the usual methods of DNAPL clean-up (slow, inefficient pumps and costly thermal treatments) to create something entirely new. “We discussed many options and in the end weren’t tied down to one idea,” explains Quinn. “And once we came up with something that worked, we sat down again and asked ourselves, ‘How can we expand this product to treat other contaminants as well?’”
While the group allowed themselves plenty of creative licenses, they were also realistic about financial parameters surrounding the project. “At the time, everything that was getting funded was nanotechnology,” Quinn explains. “We knew we had a high probability of getting our project funded if it used nanotechnology, so we decided to use nanoscale materials.” Dr. Geiger added that the nano iron particles were essential for moving EZVI deep through the soil to the highest concentrations of contamination. In order to do this without expensive trenching, they needed very small particles that could move through a series of wells.
There were also considerable regulatory hurdles surrounding the use of iron to clean up environmental pollutants because it was publicly perceived as harmful to the environment. “Every time you introduce a new technology that’s going to be released into the environment, you face a challenge in getting the regulatory community to understand that what you’re putting into the ground is going to be beneficial,” says Dr. Quinn. “Iron is in the vitamins we take and does not pose a harm to the environment in the scale and scope we were proposing to use it in.” The team came up with the idea to use food-grade products such as vegetable oil that would create a surfactant to hold the EZVI system together. “We went with materials that were specifically understood to not have an impact [on the environment],” Dr. Quinn explains. “We didn’t even test surfactants that we knew could potentially be toxic, because we knew it wouldn’t go anywhere.”
The resultant EZVI overcomes the limitations of current DNAPL treatment technologies because it is able to directly treat the contaminant particles by mimicking and therefore exploiting DNAPL’s chemical properties. The oil membrane acts as a wick that pulls the DNAPL contaminants out of the water (much as a paper towel pulls water into the towel when placed on top of a spill on your kitchen counter) while the nanoparticles break down the DNAPL into harmless compounds that can be consumed by microbes in the soil. “The EZVI droplets are like mini-reactors that are sent into the most concentrated contaminant areas,” explains Dr. Geiger. The result is a quick, effective, and cost- competitive substance that produces less toxic and more easily degradable by-products than conventional methods.
The result of the team’s flexibility and willingness to expand upon their initial hypothesis has produced a level of success that even they hadn’t initially imagined. Quinn explains that more than 60,000 gallons of EZVI has been put into the ground at four industrial locations and at three locations within the Department of Defense. The technology has won the SE Federal Labs Consortium 2005 Excellence in Technology Transfer award, the national Federal Laboratory Consortium Excellence in Technology Transfer Award for 2006, the NASA 2005 Invention of the Year Award, and the 2005 NASA Commercial Invention of the Year Award. “It was just a great experience to work with a team who knew each other so well, who had such a level of respect for each other and each others’ talents, and who weren’t afraid to pursue new ideas,” Quinn says.
Conversation with Kathleen Brooks
Kathleen Brooks joined the EZVI team while she was a graduate student and now works for NASA full time. She talked to Kerry Ellis about what she learned from the experienced team she joined.
Q: What did you learn from working on this project?
A: Even if 90 percent of your experiments fail, you just keep trying until you get one that works in the lab. In order to create something that would be reliable and work in the field, we needed to reproduce the exact same results each time, which was difficult. In this type of work, it’s important to remember that one experiment can give you results that are either false or too good to be true. You need to keep testing in order to get a product that works.
Q: How do you persist when 90 percent of your experiments fail?
A: Sometimes proving that something doesn’t work isn’t as exciting as proving that it does, but you can learn a lot from experiments that don’t work well. At the very least, you help other chemists know what not to do. You can’t expect anything to work magically the first time. None of us expected this idea to work as well as it did!
Q: Was it difficult joining a team that had worked together in the past and had such a close-knit relationship?
A: I had worked in environmental analytical chemistry for ten years, but working as a part of this team was a life-changing experience for me. I was accepted as part of the team from the beginning, and I got my job at NASA through my experience with the team. I am still part of the group today, doing research on PCBs. The work complemented my research background, so I immediately thought of ways to make the concept work. I felt comfortable and that I could just jump right in and get going.
Q: How did you get involved on this project?
A: I took an environmental chemistry class with Dr. Geiger and did a presentation on groundwork contamination. When she asked me to join the group as a researcher, I was hesitant at first. I’d focused on environmental research for so long that I felt ready to try something new. But after I spoke to Dr. Quinn and others on the team, I realized that we could really do this.
Q: What did you most enjoy about working on this team?
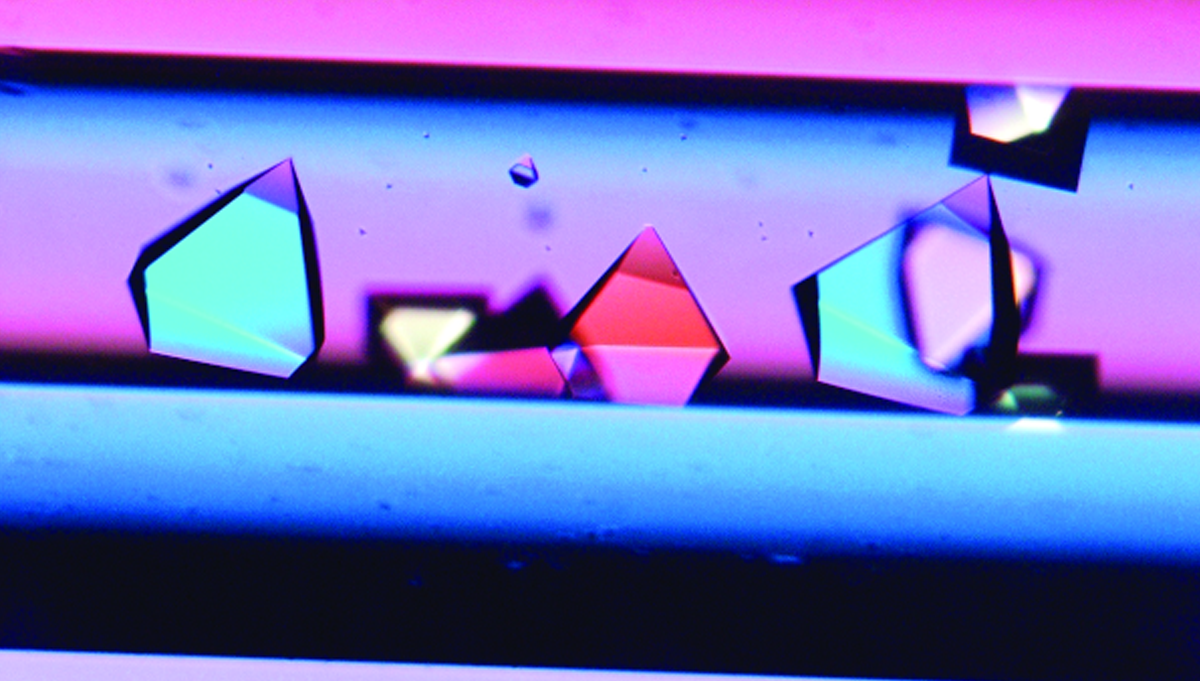
A: Everyone had very specific things to offer that made it easy to work well together. Dr. Quinn is very analytical and good at the business side of things, Dr. Clausen is an amazing think tank, and Dr. Geiger keeps us all organized. These individual contributions matched well together. Vision is also important. Dr. Clausen and the team had drawn out the basic idea, and we were able to reproduce it under the microscope. That was exciting.
Q: Do you agree with Dr. Quinn’s advice of not locking yourself in to one idea?
A: Yes. It’s a huge task to take something from a pure concept, make it work in the laboratory, and then get it into the field. For example, we had several different emulsions that worked. We preferred one version over the other options, but when we tested it in the field, our preferred choice was no longer feasible, so we were forced to go back to our previous research and continue testing.